Describe Electronegativity in Your Own Words
A o of 38 kJmol is associated with the equilibrium 2ABCD. 10 points please hurry.
Electronegativity Trend Science Trends
Describe the bond formed between two atoms with very different electronegativities.

. Use complete sentences to answer. Does an object travel farther on a smooth or slippery surface or on a rough surface. Rank the following elements from least electroneg.
In this lab you will work diligently at your own pace to answer a number of questions. D double covalent bond. The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity.
In this lab you will work diligently at your own pace to answer a number of questions. Up to 24 cash back In this atomic-level simulation you will investigate how atoms electronegativity value affects the bonds they produce. In your own words based on your text describe the basic tenets of Freudian psychoanalysis.
Find an answer to your question Briefly explain in your own words why the bond angle increases as the number of electron groups decreases. Metals have valence electrons and as a result the electronegativities of metals are Metals tend to reach stability by. Is it possible for a molecule to have a polar bond but have an overall polarity of nonpolar.
Therefore ionic bonds are very Covalent bonds are when the electrons are shared. E coordinate covalent bond. Describe how friction affected the results of your investigation.
To begin from what youve already learned about the protons and electrons in an atom what would cause an atom to have a high electronegativity value. Describe in your own words the importance and significance of water in living organisms. How is the naming of ionic and covalent compounds different.
Use a blue font to enter your answer. At what electronegativities for both Atom A and B is the bond character most likely to be covalent. What I Have Learned In your own words differentiate polar and nonpolar covalent bond.
When two atoms bond covalently one or more pair s of electrons is shared between atoms. Describe in your own words what friction is. What is electronegativity and how can it be used in determining the polarity of molecules.
In your own words describe what a Control group is. Answer the following questions directly on the document. So here we just looking to define a few times within a chemistry context to the first one we have is valence electrons.
How does electronegativity increasedecrease within a group and period in the periodic table. 15 points 1 extra credit point so you can earn up 1615. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
So these are the electrons that are most likely to be involved in any. View OL Lab 4-Ionic and Covalent Bondsasd lab 4docx from CHEM 1411 at Eastfield College. The bond becomes more ionic with two different electronegativities Describe in your own words what is meant by partial charges δ- and δ.
Electronegativity is a measure of a single atoms ability to attract the electrons shared in that bond. Describe your partner in three words. In this lab you will work to answer a number of questions.
What subatomic particles participate in chemical bonding. Propose another experiment that could be used to demonstrate that friction is a contact force. What are the partial charges at these electronegativities.
27 6 points In your own words describe the concept of electronegativity. The repulsion between electron pairs increases with an increase in electronegativity of the central atom and hence the. Your answers should be in your own words and reflect your own work.
Using just a few words have them describe your best qualities andor characteristics. It is a dimensionless property because it is only a tendency. Electronegativity is a measure of a single atoms ability to hoard electrons shared in that bond.
Because oxygen has a higher electronegativity compared to hydrogen atoms and is able to strongly attract the electrons and the polarity of water is due to the orbit having the. Science Chemistry QA Library a Describe in your own words the relationship between standard change in Gibbs free energy associated with a chemical process o and an equilibrium constant Keq. What is the meaning of the term electronegativity in your own words.
If A and B are initially present in 1M concentration find the equilibrium concentrations of all species. Be sure your answer has a unit symbol if. It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs.
Electronegativity is a measure of a single atoms ability to hoard electrons shared in that bond. When two atoms bond a pair of electrons is shared between atoms. Charming indicates they are elegant and sophisticated.
Use large carbon base molecules to determine its solubility in water. In your own words explain what the Partial Charges and Bond Character button display. In your own words describe what an experimental group is.
In your own words describe how you can use the structure of a large carbon-based molecule to determine its solubility in water. Include in your response the unique properties associated. Noun the tendency or a measure of the ability of an atom or molecule to attract electrons and thus form bonds.
Electronelectrons are transferred to the anion with the greater electronegativity. From WordNet 30 Copyright 2006 by Princeton University. As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements.
I know my partner was given to me by God. Using a periodic table use the one in the chapter 2 module how many protons neutrons and electrons does Fluorine F. BIU A А IX EX X SE 12pt Paragraph Next Previous.
SOLVEDIn your own words define the following terms. Covalent bonds do have strength but not as much as ionic bonds. The atoms that share these electrons do not vary in electronegativity as much as ionic bonds.
Differentiate bonding and non-bonding electrons. Lastly explain why the noble gases are not assigned electronegativity values. Noun chemistry the tendency of an atom or radical.
When moving from left to right across the periodic table electronegativity increases with the exception being the noble gases. Describe the periodic trends in electronegativity. In general electronegativity decreases as you move down a group in the periodic table this correlates.
Sulfide required to make 3kg of copper.

Electronegativity Chart Chemistry Lessons Chemistry Classroom Teaching Chemistry

Electronegativity Chart Periodic Table Chemistry Lessons Chemistry Basics
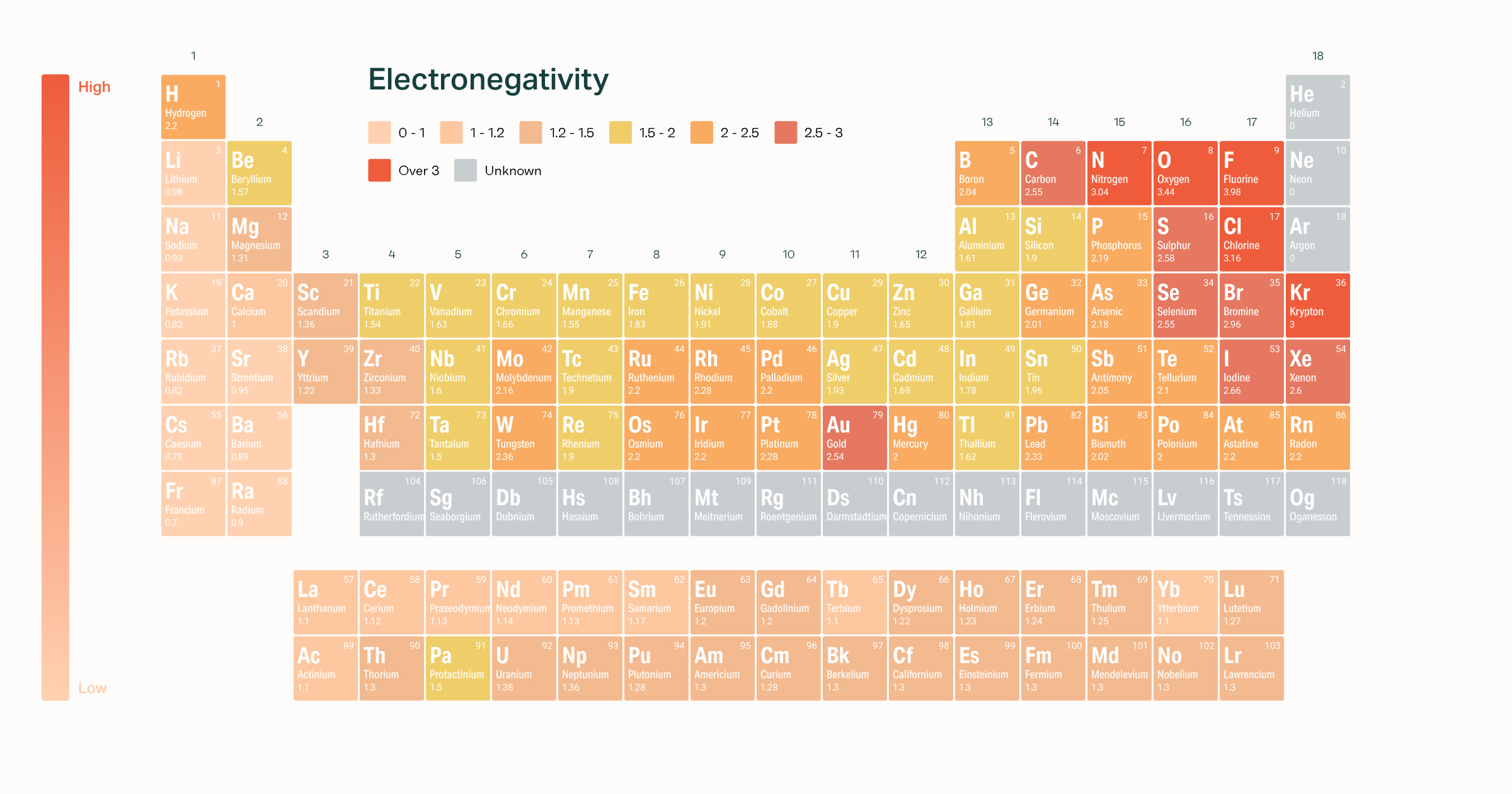
Electronegativity Of The Elements

What Is Electronegativity Trends Example Variation

What Is Electronegativity Trends Chart Periodic Table Chemtalk
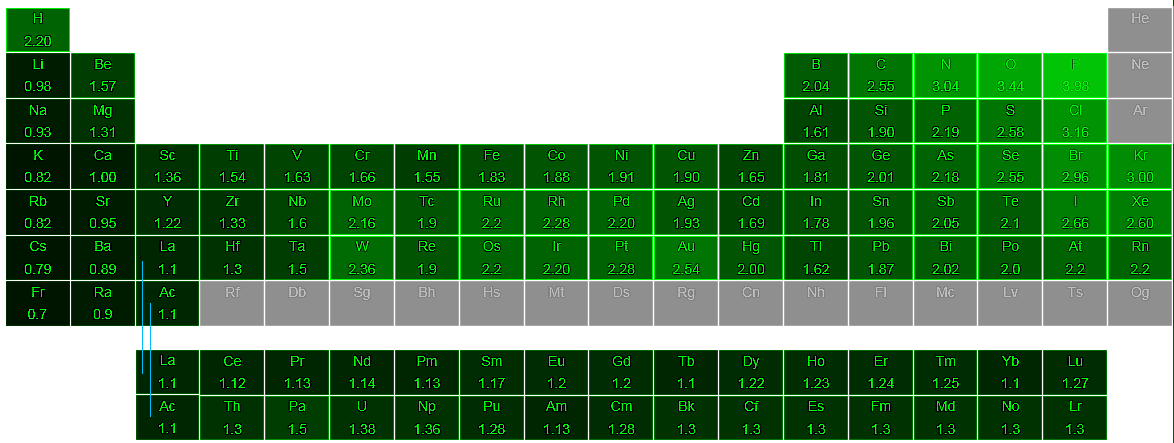
Definition Of Electronegativity Chemistry Dictionary

Printable Periodic Tables Science Notes And Projects Periodic Table Words Periodic Table Printable Science Notes

Electronegativity Examples Trends Video Lesson Transcript Study Com

No comments for "Describe Electronegativity in Your Own Words"
Post a Comment